In this page
Plane polarised light
Asymmetry
Meso compound
Centre of symmetry
Racemic mixture
Plane polarised light
Ordinary light is circularly polarised when it is passed through a device called Nicol prism its plane of polarisation is restricted to one plane hence, it is called plane polarised light.
Stereo centre:
A carbon atom which has four different groups attached to it. A molecule with a stereo centre will not have a plane of symmetry and will be optically active. Stereo centre is also referred to as chiral centre/chiral carbon, or asymmetric carbon.
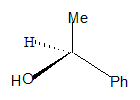
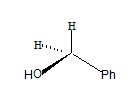
All the groups around the central carbon are not different, hence it is not a stereo centre. The molecule has a plane of symmetry
Asymmetric carbon:
A carbon with four different groups Same as stereo centre.
Note: an atom cannot be asymmetric, here a carbon atom with four different groups is labelled as an asymmetric carbon.
Asymmetry
Absence of all elements of symmetry. An asymmetric molecule will be optically active. It will have one more molecule which is it's non superimposable mirror image, they are termed a pair of enantiomers.
Dissymmetry
is the property of non superimposability on its mirror image, a dissymmetric molecule may have a simple axis of symmetry yet it will be optically active and exist as a pair of enantiomers.
Note: Both asymmetric and dissymmetric molecules are optically active.
Chirality
is derived from the greek word chiral meaning handedness (property of non superimposability on its mirror image) . A chiral molecule is optically active and exists as a pair of enantiomers.
Meso compound
has two are more stereo centers, still is optically inactive since it has a plane of symmetry.
Plane of symmetry
an imaginary plane which passes through atoms and bonds in a molecule so that it divides it into two halves each one a mirror image of the other.
Simple axis of symmetry
A "n" fold simple axis of symmetry is an axis passing through a molecule in such a way that rotation about this axis by an angle of 360/n degrees brings the molecule into a position indistinguishable from the original position.
Centre of symmetry
A point within the molecule such that if a straight line is drawn from any part of the molecule through it and extended on either side to an equal distance meets with similar environment. For a sphere the geometrical centre represents the centre of symmetry.
Alternating "n" fold axis of symmetry(Rotation-reflection symmetry)
It is an axis such that the molecule containing such an axis is rotated by 360/n degrees about the axis and then reflection is effected across a plane at right angles to the axis a new molecule is obtained which is indistinguish able from the original one.
dextro or laevo rotatory
An optically active molecule rotates the plane of polarization of polarized light to the right it is considered to be “dextro” rotatory, if it is to the left it is “laevo” rotatory. dextro and laevo rotation is also indicated with the sign (+) and (-) or “d” and “l” letters respectively.
Racemic mixture
An equal mixture of a pair of enantiomers will be optically inactive, since they have opposiste rotation and of the same value they cancel each other. Such a mixture is a raecemic mixture.
Resolution
The process of separation of a racemic mixture into the individual pure enantiomers.
Racemisation
a pure enantiomer becomes a mixture under the influence of heat,light acids, or bases.