
07-11-1888 : 21-11-1970
Noble Prize in Physics 1930
''Inelastic scatterng of light"
When molecules are allowed to interact with monochromatic light a portion of the light is scattered. The frequency of most of the scattered radiation (~99%) is the same as that of the incident radiation. This is known as the Rayleigh scattering. A small portion of the scattered radiation is found to have different frequencies and the corresponding spectral lines are termed as the Raman lines.The differences in energy between these scattered radiations and the original incident radiation are characteristics of the irradiated molecule and are identical with the energy differences between certain vibrational and rotational levels of the molecule. Since the incident and scattered radiations are identical,this type of scattering is termed as elastic scattering. But in Raman scattering a part of the energy of the incident light is used in exciting the molecule to the immediate higher energy level and hence the radiation emitted by the molecule will be correspondingly of lower energy and hence lower frequency (Stokes lines).On the other hand, some of the molecules which are already in an excited level , give their excess energy to the incident radiation and move into the immediate lower energy level. Hence the emitted radiation (scattered radiation) will have higher energy than the incident radiation.This results in the anti-stokes lines. Since the energies of the incident and emitted radiation are different, the collision between the molecules and the incident radiation is said to be inelastic. The Rayleigh and Raman scatterings can be understood clearly from the following diagram.

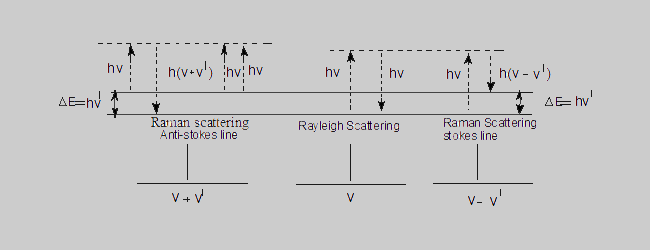

Rayleigh scattering The momentarily excited molecule returns to the original level, the frequency of the emitted radiation is the same as the incident radiation.
Raman scattering The excited molecule returns to an energy level which is different from the original level.
Stokes lines The energy of the scattered radiation is less than the incident radiation.
Anti-Stokes lines The energy of the scattered radiation is more than the incident radiation.
In general, the Stokes lines are stronger than the anti-Stokes lines since molecules prefer to stay in the lower vibrational state.