
Inductive Effect
Consider the C-C bond as in ethane.
The pair of electrons binding the two atoms is equally shared.
Consider the C-Cl bond, due to the greater electronegativity of Cl there is polarisation( shift ) of electron density towards Cl, as a result the bond becomes polarised with partial negative charge on Cl and partial positive charge on C .
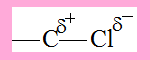
Now consider 1-Chloropropane.
C1 has a partial positive charge due to -Cl, as a result the electron density between C1 and C2 is polarised towards C2 and therefore C2 acquires some positive character though not as much as C1. This polarization of electron density across the carbon chain due to the presence of certain groups is Inductive effect.
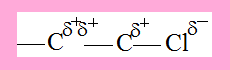
Inductive effect is prominent at C1 and to a lesser extent at C2 but is insignificant beyond.
Atoms or groups which pull electron density towards themselves are considered as exhibiting negative Inductive effect (-I) and groups which push electron density away from themselves exhibit positive Inductive effect (+I).
(-I) groups make the carbon atom bonded to them electrophilic in character. Hence a nucleophile can easily form a bond with it.
Halogens are responsible for –(I) effect. Alkyl groups exhibit (+)I effect.
The decreasing order of Inductive effect of some alkyl groups are