
Consider the following reactions,
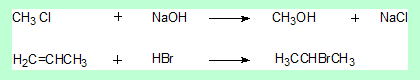
- The first is a substitution reaction at the saturated carbon (sp3).
- The carbon in the product has the same hybridisaion state as the reactant. There is no stereochemical change.
- The second is an addition reaction. The central carbon of propene changes its hybridisation state from sp2 to sp3.
- But that carbon either in the reactant or the product is not a stereo centre. Again there is no stereochemical change.
Now consider the following reaction.
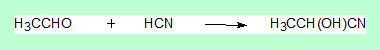
Here the sp2carbon of the aldehyde becomes a sp3 carbon and more importantly it becomes a stereo centre. So what?
Well a compound with one stereo centre can exist as a pair of enantiomers, with the configuration as either ( R ) or ( S ) and the compound can be either dextro or laevo rotatory. Now the question is, in the above reaction what is the configuration of the product? Is it (R) or (S)? Well experimentally it has been observed to be a mixture (racemic mixture). In organic synthesis, When a sp2carbon is converted to sp3, the product formed is always a racemic mixture. One more point let us look at the addition reaction once again, yes here a change in hybridsation state has occured, yet the product is not a racemic mixture, because it is not optically active, so the statement made has to be modified as follows. When a sp2 carbon is converted to sp3 the product formed is always a racemic mixture provided the carbon concerned in the product has become a stereo centre.
Why a racemic mixture? Why not only one of them?
The reason will be clear when we consider the mechanism of the reaction.