
Alkyl halides-Mechanism of nucleophilic substitution Level 2
Exercise 2
1.
Alkyl halides can be prepared from alcohols by using PCl5 or SOCl2 (Thionyl chloride). SOCl2 is preferred though more toxic and expensive because
a) the yield is good.
b) the side products are gases so, the product can be isolated easily.
c) the product can be obtained in high purity.
d) all of the above reasons.
2.
Which one of the following statements is true concerning a good leaving group?
a) No influence on either of them.
b) It promotes a SN1 pathway.
c) It promotes a SN2 pathway.
d) It promotes SN1 & SN2
3.
Which of the following compounds would undergo nucleophilic substitution under normal conditions? 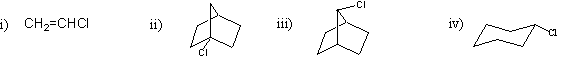
a) only i) and ii)
b) only ii) and iii)
c) only iii) and iv)
d) only i) and iv)
4.
Which of the following is not true concerning (S)-2-Chlorobutane?
a) SN2 reaction with NaOH results in inversion in configuration.
b) Dehydrohalogenation results in a product which is optically inactive.
c) It is dextro-rotatory because the configuration is S.
d) It is the product of addition of HCl to 1-Butene
5.
(R)-2-Methylbutanol is heated with conc. HCl, which one of the following is the most likely product?
a) (R)-2-Methyl-1-Chlorobutane
b) (S)-2-Methyl-1-Chlorobutane
c) (±)-2-Methyl-1-Chlorobutane
d) (+)-Isopentylchloride
6.
In vinyl and aryl halides, nucleophilic substitution of the halogen is not possible under normal conditions because
a) the lone pair of electrons of chlorine atom is involved in delocalization.
b) the C-Cl bond is stronger than the corresponding bond in an alkyl halide.
c) carbon attached to the halogen atom is weakly electrophilic.
d) all of these
7.
Which of the following conditions does not favour (does not make much of a difference) a SN1 mechanistic pathway in a nucleophilic substitution reaction?
a) bulky groups at C with the nucleofuge.
b) a strong nucleophile
c) polar solvent
d) a good leaving group.
8.
Which solvent type is best suited for the reaction shown? CH3(CH2)15CH2Br + NaCN --------> CH3(CH2)15CH2CN + NaBr
a) Polar
b) non polar
c) polar protic
d) polar aprotic
9.
Of the following solvents which is best suited for a SN2 reaction?
a) Ethanol
b) Hexane
c) DMSO
d) water
10.
Which one of the following compounds undergoes solvolysis in aqueous ethanol most rapidly?
a) 2HC=CHCl
b) H2C=CHCH2Cl
c) CH3CH2CH2Cl
d) CH3Cl
11.
The top most point in the energy profile of a SN2 reaction represents
a) the intermediate
b) the transition state
c) activated complex
d) both b)& c)
12.
Nucleophilic substitution at the sp3 carbon linked to -OH group of an alcohol is generally not possible, because the –OH group is a bad leaving group. This can be made possible by converting the –OH group.
a) to a mesylate.
b) to a tosylate
c) to –OH2+ by protonation.
d) all of these.
13.
A SN2 reaction is
a) stereospecific
b) stereoselective
c) regioselective
d) both a) and b)
14.
A dextro rotatory alkyl halide undergoes nucleophilic substitution by SN2 mechanism, the product is
a) laevo rotatory
b) dextro rotatory
c) a racemic mixture
d) can’t be sure.
15.
For a SN2 reaction the influence on the rate of the reaction if the concentration of both alkyl halide and base reduces by half is
a) decreases by half.
b) reduces by one fourth.
c) no change.
d) increases by four times.
16.
For a SN1 reaction the influence on the rate of the reaction if the concentration of both alkyl halide and base is doubled is
a) increases by twice the value
b) increases four times
c) no change.
d) increases by eight times
17.
1-Chloro-2,2-dimethylpropane undergoes nucleophilic substitution by SN1 mechanism even though it is a primary alkyl halide because
a) reaction involves a tertiary carbocation.
b) it can happen only in DMSO
c) it cannot substitute by SN2
d) low T is favourable.
18.
Benzyl chloride because of the –CH2Cl is a primary alkyl halide, yet it undergoes nucleophilic substitution predominately by the SN1 mechanism because:
a) the intermediate is benzyl carbocation which is stabilised through resonance.
b) the carbon chlorine bond is highly ionic.
c) The intermediate formed is a tertiary carbocation.
d) of steric factors.
19.
When 4-Chloro-2-methyl-2-butene undergoes solvolysis in ethanol the product will be
a) 4-Ethoxy-2-methylbutene
b) 3-methyl-3-ethoxybutene
c) 2-methyl-3-ethoxybutene
both a) and b)
20.
In a nucleophilic substitution the rate data was collected and tabulated. Which of the following is consistent with a SN1 mechanism?
i) [R-X] = 0.10, [base] =0.001, r =1.3 x 10-2
ii) [R-X] = 0.20, [base] =0.001, r =2.6 x 10-2
iii) [R-X] = 0.10, [base] =0.002, r=1.3 x 10-2
iv) [R-X] = 0.30, [base] =0.003, r =3.9 x 10-2
a) ii) & iv)
b) ii) & iv)
c) ii) & iii)
d) all of them.
21.
t-BuCl is solvolysed faster in 90% H2O-10% dioxane than in 90% D2O-10% dioxane.This is because:
a) The polarity of D2O is greater than H2O.
b) D2O promotes SN2 pathway
c) The intermediate carbocation is less stable in D2O due to decrease in hydrogen bonding interactions.
d) The intermediate carbocation is more stable in D2O due to increase in hydrogen bonding interactions.
22.
Use of which one of the following promotes a SN1 mechanism?
a ) an aprotic solvent like DMSO
b) Phase transfer catalyst
c) Crown ethers
d) Ag+
23.
2-Chlorobutane is treated with KI and acetone, the product formed is:
a) 2-Butene
b) (S)-2-iodobutane
c) (R)-2-Iodobutane
d) (±)-2-Iodobutane
24.
(S)-1-Bromo-2-Butanol is reduced with Zn / H+. The product is:
a) (R)-2-Butanol
b) (S)-2-Butanol
c) (±)-2-Butanol
d) Butane
Copyrights: 2005 www.chemvista.org All Rights Reserved