Sigmatropic rearangements
The Huckel Mobius theory
Ms. Preeti.
- Sigmatropic rearrangement is a type of pericyclic reaction that goes through a cyclic transition state.
- It involves migration of a sigma bond next to a π system to a new position in the molecule.
- A sigmatropic shift involves shifting of an atom or a group to a new position in the molecule.
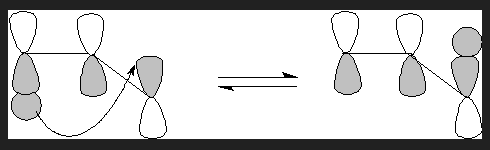
In the following example a Hydrogen atom migrates from position 1 to 3. Hence it is termed a [1,3]-shift.
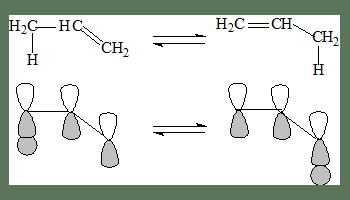
In the following, the shift is from 1 to 5, and it is termed as a [1,5]-shift.
Examples of this type are [1,j]- shifts. 1 is the origin and j is the terminus of the shift.
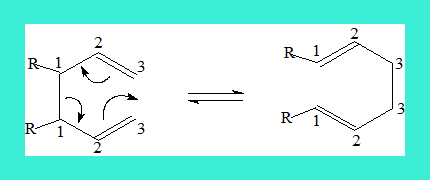
Similarly there are [i,j]-shifts or rearrangements. When a sigma bond migrates through the Pi system to a new position both ends of the migrating sigma bond is numbered 1 and 1 and the ends of the new sigma bond are given an identical but appropriate number as shown in the following example. The name includes the position of the new sigma bond, thus the following is a [3,3]-sigmatropic rearrangement. Such a rearrangement is also termed Cope rearrangement.
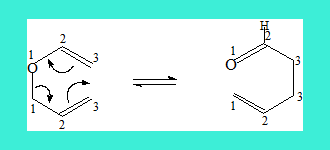
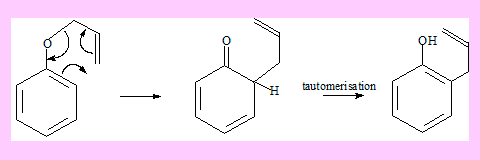
A similar reaction wherein an allyl vinyl ether reacts is termed Claisen rearrangement. Here is an example.
The rearrangement of allyl aryl ethers is also considered as Claisen rearrangement. In this case the final product is formed involving another step through tautomerisation. The driving force for this step is the stability of the product which is aromatic.
The shifts can take place in two different modes. If the transfer occurs on the same side of the π system it is called suprafacial shift and it is geometrically feasible.
A suprafacial 1,3-shift
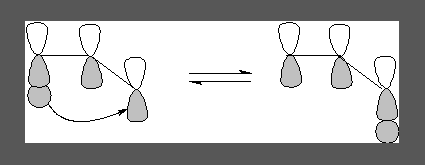
A antarafacial 1,3-shift
If the transfer occurs to the opposite lobe on the top it is termed an antarafacial shift.
In this case the 1s-orbital lobe is the smallest and distance with the interacting lobe to which transfer occurs is maximum, most importantly the interaction is not effective as can be seen in the following diagram, the molecule has to, kind of twist for this to occur and rotation here is restricted. Geometrically this kind of shift is not feasible.