
Iodoform test answers
1. Give the structure of compounds which give a positive Iodoform test and have the following characterisitics.
(An aldehyde or ketone which has a methyl group adjacent to the carbonyl group will give a positive test.)
An aldehyde
CH3CHO
Two ketones
C2H5COCH3
CH3COCH3
An aromatic ketone
C6H5COCH3
An alcohol with four carbon atoms
CH3CHOHCH2CH3
2) Give the structure of compounds which do not give a positive Iodoform test and have the following characteristics.
a) An aldehyde.
HCHO
b) A cyclic ketone
Cyclohexanone
c) An aromatic aldehyde
C6H5COC6H5
3) Give reasons
a) 3-Pentanone has a methyl and a carbonyl group,
still does not give a positive Iodoform test.
still does not give a positive Iodoform test.
This is because the methyl group and the carbonyl groups should be next to each other, which is not the case in 3-Pentanone ( CH3CH2COCH2CH3)
1-Propanol does not give a positive Iodoform test.
The group necessary should be CH3CHOH-. In 1-Propanol this group is not there (in 2-Propanol this group is there and hence it gives a positive test)
Formation of Iodoform can be used as a test to
detect a methyl ketone while formation of
chloroform cannot be used.
detect a methyl ketone while formation of
chloroform cannot be used.
Iodoform is a yellow solid and can be observed in the test while chloroform is a colourless liquid and cannot be noticed.
Write the equations for the preparation of the following carboxylic acids through haloform reaction.
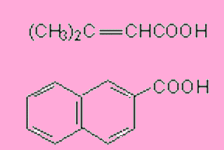
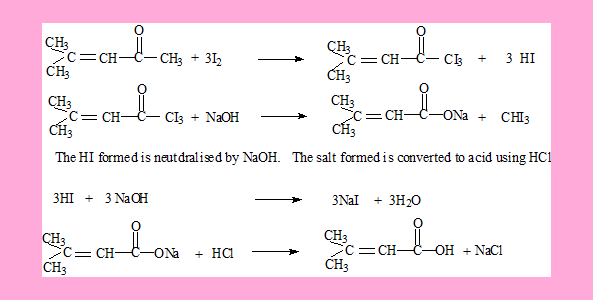
Answers
The second compound.
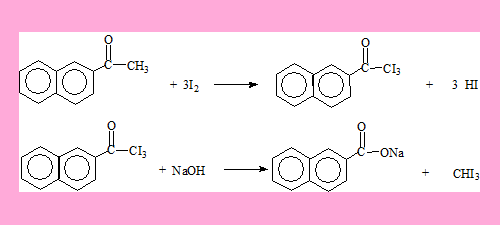
The HI formed is neutralised by NaOH. Also the acid can be generated from its carboxylate salt by using HCl.
Copyrights: 2005 www.chemvista.org All Rights Reserved