
Stereochemistry of addition of Bromine to Cis and Trans-2-Butene
Bromine adds on to the cis isomer giving rise to (±)-2,3-Dibromobutane while the trans isomer leads to meso-Dibromobutane exclusively.
These results are consistent with the two step mechanism with bromonium ion intermediate.
The carbocation intermediate would have given rise to the meso-Dibromo compound as well in the case of addition involving the cis isomer. This is because the nucleophile could have approached the carbon from either side(the carbon with the positive charge is sp2 hybridised and therefore planar). The absence of meso-product in this reaction is in support of the two step and bromonium ion intermediate mechanism.
The formation of different products can be understood by visualising the coresponding intermediates
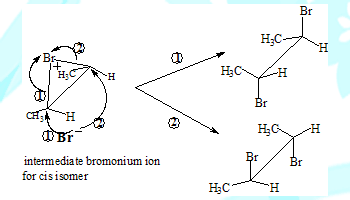
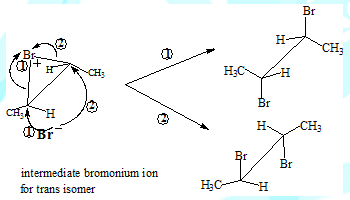
Some salient features
Cis-2-Butene leads to (±)-2,3-Dibromobutanes
Trans-2-Butene leads to meso-2,3-Dibromobutane
The reaction is stereo specific. (See terms in stereochemistry)
A molecule with "n" stereocentres can have 2n stereo isomers.2,3-Dibromopentane has two stereo centres and as expected exists as four stereo isomers. The groups at each stereo centre are not identical.
2,3-Dibromobutane also has two stereo centres and should have four stereo isomers, but has only three because the groups at each stereo centre are identical this leads to the existence of a meso compound.
Meso compounds are also possible in compounds with more than two stereo centres.